Affiliate Disclosure: As an Amazon Associate we earn advertising fees from qualifying purchases. learn more.
With temperatures on the rise and natural resources dwindling, clean energy is a leading worldwide concern. One of the most common sources of clean electricity, in particular, is solar panels. What is it, and how does it work?
What is a Solar Panel?
Solar panels are made up of photovoltaic cells, which are two semiconductors lying on top of each other. They work by knocking off an excess electron, which is pulled away by a metal plate that carries it to wires leading to the DC/AC converter when a photon strikes the topmost semiconductor.
While there are several forms of solar power, including photovoltaic, solar thermal, and concentrated solar power (CSP), we will only focus on the first, as it is the only form that involves solar panels (thermal uses tubes that collect radiation, CSP uses mirrors).
This article will cover how traditional photoelectric solar panels collect energy and how that energy is converted to usable electricity that flows into our power lines.
How Does A Solar Panel Work?
Solar panels work based on the photovoltaic effect. Solar panels, which are also called photovoltaic (PV) panels, use the photovoltaic effect to turn sunlight into electricity. This process is based on the unique properties of certain materials, like silicon-based semiconductors. These special silicon-based semiconductors produce an electric current when exposed to sunlight. The photovoltaic effect happens when light particles called photons and charged particles called electrons meet in the solar panel’s material.
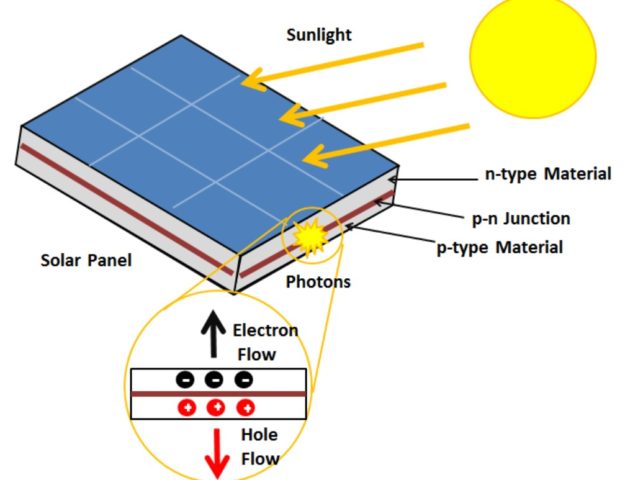
These photovoltaic effects happen in five different stages:
Absorption: Sunlight, which is made up of photons with different amounts of energy, hits the solar panel and reacts with the semiconductor material. The bandgap energy of the semiconductor material is picked with care so that it can capture photons from the sunlight spectrum.
Excitation: When an electron in a semiconductor material absorbs a photon, the photon gives the electron energy. This causes the electron to move from a lower energy state (called the “valence band”) to a higher energy state (called the “conduction band”). This makes a pair of an electron and a positively charged hole. The electron goes to the conduction band, leaving a positively charged hole in the valence band.
Charge separation: An electric force inside the semiconductor material helps separate the electron and the hole. The electron with a negative charge goes towards the back of the solar panel, while the hole with a positive charge moves towards the front.
Collection: Conductive metal wires are connected to the front and back of the solar panel. These plates collect the split charges, which create a flow of electrons that can be used as an electric current. The front electrode gets the electrons from the conduction band, and the back electrode gets the holes from the valence band.
Circuit completion: When electrons pass through an external circuit, they create an electric current that can be used to power devices.
Solar panels are composites of smaller photovoltaic cells, which are 2 semiconductors layered on top of each other. Photovoltaic cells need to be structurally stable and electrically conductive. Silicon and phosphorus help achieve those qualities.
Silicon has 4 valence electrons, which allows it to form many stable bonds. Most critically, it is able to form stable bonds with itself and make a rigid grid of atoms. These silicone grids are the basis of many rocks and computer chips.
If the grid is pure silicon, each silicon atom will have all 4 of its valence electrons paired and bonded, and none of them will be able to move around. Because of that, the grid will not be electrically conductive since electricity is, by definition, the movement of charged particles.
However, if you replace some of the silicon atoms in the grid with atoms that have 5 valence electrons, only 4 of them will bond and attach to the grid and 1 will be left free to move around. Those free electrons will now be able to carry an electric charge, and the grid will now be an N-type semiconductor. Solar panel semiconductors usually have their silicon seeded with phosphorus.
Conversely, if you replace some silicon atoms with an element that has only 3 valence electrons, it will not be able to bond to the 4th of its neighboring silicon atoms. This missing electron causes an overall positive charge in the grid and makes it a P-type semiconductor. This is achieved by seeding the silicon grid with boron.
If you layer these semiconductors on top of each other, the extra electrons from the N-type semiconductor will try to migrate to the empty spaces in the P-type semiconductor in an attempt to balance out the negative and positive charges and make the two grids stable again. This creates an electric field between the two.
The electrons cannot migrate on their own; however, the distance between the semiconductors is purposefully made too big. They need a light particle, a photon, to knock into the N-type semiconductor and give the extra electrons enough energy to free themselves from it.
However, before they can fill in the spaces in the P-type conductor, the electrons find themselves pulled away by two conductive metal plates on the side of the cell. From there on, those electrons, now a part of a Direct Current, must be converted to Alternating Current.
Direct and Alternating Current
The electric current coming out of the solar panels is direct current, which is rarely used in houses because it requires many steps to transform and make usable. Therefore, before being sent into the house, a solar inverter converts the electricity to an alternating current. From there, power flows onto the house as usual.
How Can a Solar Panel be Constructed?
Solar panels usually are constructed with 6 layers. From the top down, Solar Panels have:
- An aluminum frame, which holds the rest of the layers together and protects them.
- A sheet of tempered glass, non-reflective, to make sure that no valuable photons are wasted. The glass is tempered, meaning that it is 4 times stronger than regular window glass. This is important for solar panels, as they are hung outdoors. They must withstand strong winds and rain without breaking. Also, rather than breaking into large sharp shards, the tempered glass breaks into tiny, relatively harmless pieces.
- An EVA encapsulant. Ethyl Vinyl Acetate (EVA) prevents water and dirt from getting into the solar cells and protects it from shock. Encapsulants must be durable, transparent, and flexible.
- The photovoltaic solar cells are in the center of the sandwich, with the N-type semiconductor in front and the P-type behind it.
- 2nd layer of EVA encapsulant.
- The back panel stabilizes the whole structure and provides a base for it to lie on.
Since silicon can make many different structures, there are a few ways of creating silicon cells:
- Monocrystalline cells are made by cutting out silicon wafers from a big block of black silicon and then affixing them to a solar panel. They are the most efficient type of cells.
- Polycrystalline cells consist of many silicon cells melted together to form one panel. They look blueish and are cheaper than monocrystalline cells.
- Amorphous solar cells are non-crystalline, and so are flexible and can be paper-thin. They are attached to glass or metal but are unfortunately very inefficient.
- How Long Do Solar Panels Last?
- What is a Solar Panel & How do they Work?
- Are Fossil Fuels Used to Make Solar Panels?
- How Much Do Solar Panels Cost & Are They Worth It?
- How Many Solar Panels Are Needed to Run An AC?
- How Much Electricity Does a Solar Panel Produce?
- Types of Solar Panels Explained – Must Read Before Buying
- Do Solar Panels Keep the Roof Cool?
- How to Clean Solar Panels? : A Step By Step Guide
- How Many Solar Panels to Run a TV?
The History of Solar Panels
Becquerel
The history of the photovoltaic cell is the history of the photovoltaic effect: that effect, which frees electrons and creates an electric field when a semiconductor is hit by the sun. The photovoltaic effect was discovered in 1839 by Edmond Becquerel when he was only 19. He was in his father’s laboratory when he coated a platinum electrode with silver bromide. When the device was illuminated, it generated an electric field.
Einstein
The photovoltaic effect was investigated in greater detail by none other than Albert Einstein. In 1905, he published a paper explaining exactly why only photons of certain energy can create the effect and why the frequency and not the amount of light shined on the surface created the effect.
He did it using Planck’s then-revolutionary theory that light is a particle – a photon – and not a wave. He won a Nobel prize for it in 1921.
Bell Labs and Beyond
In 1954, a group of 3 scientists created the first solar cell. Calvin Fuller and Gerald Pearson worked on semiconductors to be used in computers, and David Chapin worked on creating a power source that could power telephones in the desert. The three joined together under Bell Labs to put the photoelectric effect to good use.
These 1954 Bell Labs solar cells had an efficiency of about 4% and eventually grew to about 11% efficiency. Hoffman Electronics outdid their record in 1960 with a 14% efficiency cell. Solar energy kept on evolving until today when most solar cells have an efficiency of about 20%.
- Checkout the Best Flexible Panels 2021
Final Thoughts about Solar Panels
Solar energy is a technology that has only gotten better with age: from the first 1% efficiency, photovoltaic cells evolved into the modern 20% efficiency solar panel. Modern solar panels are made of 6 layers, with an aluminum frame, tempered glass, EVA encapsulants, photovoltaic cells, and back panel.
The solar panel depends on the photovoltaic cell, whose 2 semiconductors create an electric field when hit by the sun’s photons. The free electrons are then taken up by conductive metal plates, which bring them to the solar inverters, which turn them into Alternating current suitable for use in the house.
With any luck, solar energy will become even more popular in the coming years, and further developments will happen in its technology.
External Sources:
- LiveScience – How do solar panels work
- https://www.hindawi.com/journals/jnp/2013/953153/
- Absorption of Light
- Popular Mechanics – How do solar panels work
- Byju’s – Einstein’s explanation of the photoelectric effect
- https://en.wikipedia.org/wiki/Electron_hole
- https://www.physicsclassroom.com/class/circuits/Lesson-2/Requirements-of-a-Circuit